Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 SO
SO and HF/HCl solutions into toluene with Aliquat336; Sulfate and fluoride complex formation of Mo and W towards chemical studies of seaborgium (Sg)
and HF/HCl solutions into toluene with Aliquat336; Sulfate and fluoride complex formation of Mo and W towards chemical studies of seaborgium (Sg)Toyoshima, Atsushi; Mitsukai, Akina; Tsukada, Kazuaki; Oe, Kazuhiro*; Haba, Hiromitsu*; Komori, Yukiko*; Murakami, Masashi; Kaneya, Yusuke*; Sato, Daisuke*; Asai, Masato; et al.
Journal of Radioanalytical and Nuclear Chemistry, 317(1), p.421 - 430, 2018/07
Times Cited Count:3 Percentile:25.46(Chemistry, Analytical)We have studied extraction behavior of group-6 elements Mo and W to search for suitable conditions for an on-line extraction experiment of their heavier homolog, seaborgium (Sg). Batch-wise extraction of carrier-free radiotracers 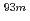 Mo and
Mo and 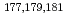 W were carried out from 0.10 - 8.6 M H
W were carried out from 0.10 - 8.6 M H SO
SO and 1.0
and 1.0 10
10 - 5.0 M HF/1.0 M HCl into toluene with a quaternary ammonium compound, Aliquat336. Anionic sulfate complexes of Mo and W with charge - 2 were extracted with Aliquat336 from H
- 5.0 M HF/1.0 M HCl into toluene with a quaternary ammonium compound, Aliquat336. Anionic sulfate complexes of Mo and W with charge - 2 were extracted with Aliquat336 from H SO
SO solutions with concentrations of [H
solutions with concentrations of [H SO
SO ]
]  2 M. In HF/1.0 M HCl, oxyfluoro complexes of Mo and W with charge - 1 were interpreted to be formed and extracted with Aliquat336. From these results, favorable conditions for the extraction of Sg are discussed.
2 M. In HF/1.0 M HCl, oxyfluoro complexes of Mo and W with charge - 1 were interpreted to be formed and extracted with Aliquat336. From these results, favorable conditions for the extraction of Sg are discussed.
Komori, Yukiko*; Yokokita, Takuya*; Kasamatsu, Yoshitaka*; Haba, Hiromitsu*; Toyoshima, Atsushi; Toyomura, Keigo*; Nakamura, Kohei*; Kanaya, Jumpei*; Huang, M.*; Kudo, Yuki*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1385 - 1388, 2015/02
Times Cited Count:2 Percentile:16.04(Chemistry, Analytical)We studied solid-liquid extraction behavior of carrier-free Mo and W radiotracers onto an Aliquat 336-loaded resin from HCl solutions toward extraction chromatography experiments of element 106, seaborgium(Sg). Distribution coefficients (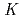
 ) of Mo and W on the resin were determined as a function of HCl concentration by a batch method. On-line extraction chromatography of Mo and W was also carried out in 2-8 M HCl solutions with an automated rapid chemistry apparatus. The order of extraction probability from 2 to 8 M HCl was Mo
) of Mo and W on the resin were determined as a function of HCl concentration by a batch method. On-line extraction chromatography of Mo and W was also carried out in 2-8 M HCl solutions with an automated rapid chemistry apparatus. The order of extraction probability from 2 to 8 M HCl was Mo W, which reflected the order of the
W, which reflected the order of the 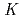
 values in the batch experiment.
values in the batch experiment.
Even, J.*; Yakushev, A.*; D llmann, Ch. E.*; Haba, Hiromitsu*; Asai, Masato; Sato, Tetsuya; Brand, H.*; Di Nitto, A.*; Eichler, R.*; Fan, F. L.*; et al.
llmann, Ch. E.*; Haba, Hiromitsu*; Asai, Masato; Sato, Tetsuya; Brand, H.*; Di Nitto, A.*; Eichler, R.*; Fan, F. L.*; et al.
Science, 345(6203), p.1491 - 1493, 2014/09
Times Cited Count:67 Percentile:82.96(Multidisciplinary Sciences)A new superheavy element complex, a seaborgium carbonyl, has been successfully synthesized, and its adsorption property has been studied using a cryo-thermochromatography and  -detection apparatus COMPACT. Nuclear reaction products of short-lived
-detection apparatus COMPACT. Nuclear reaction products of short-lived  Sg preseparated with a gas-filled recoil ion separator GARIS at RIKEN were directly injected into a gas cell filled with He/CO mixture gas, and chemical reaction products of volatile carbonyl complexes were trasported to COMPACT. The Sg carbonyl complex detected with COMPACT was found to be very volatile with adsorption enthalpy of
Sg preseparated with a gas-filled recoil ion separator GARIS at RIKEN were directly injected into a gas cell filled with He/CO mixture gas, and chemical reaction products of volatile carbonyl complexes were trasported to COMPACT. The Sg carbonyl complex detected with COMPACT was found to be very volatile with adsorption enthalpy of 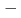 50 kJ/mol, from which we have concluded that this complex should be a Sg hexacarbonyl Sg(CO)
50 kJ/mol, from which we have concluded that this complex should be a Sg hexacarbonyl Sg(CO) . This is the first synthesis of organometallic compounds of transactinide elements for which only simple inorganic comounds have been synthesized so far.
. This is the first synthesis of organometallic compounds of transactinide elements for which only simple inorganic comounds have been synthesized so far.
Br chle, W.*; Andrassy, M.*; Angert, R.*; Eberhardt, K.*; Fricke, B.*; Gregorich, K. E.*; G
chle, W.*; Andrassy, M.*; Angert, R.*; Eberhardt, K.*; Fricke, B.*; Gregorich, K. E.*; G nther, R.*; Hartmann, W.*; Heimann, R.*; Hoffman, D. C.*; et al.
nther, R.*; Hartmann, W.*; Heimann, R.*; Hoffman, D. C.*; et al.
1st International Conference on the Chemistry and Physics of the Transactinide Elements; Extended Abstracts, 4 Pages, 1999/00
no abstracts in English
Tsukada, Kazuaki
Kagaku To Kogyo, 51(4), P. 615, 1998/00
no abstracts in English
M.Schaedel*; W.Bruechle*; E.Jaeger*; B.Schausten*; G.Wirth*; W.Paulus*; R.Guenther*; K.Eberhardt*; J.V.Kratz*; Seibert, A.*; et al.
Radiochimica Acta, 83(3), p.163 - 165, 1998/00
no abstracts in English
Otani, Ryo; Sato, Tetsuya; Aoki, Ryota*; Shirai, Kaori*; Suzuki, Hayato; Tsukada, Kazuaki; Asai, Masato; Ito, Yuta; Nagame, Yuichiro*; Sakama, Minoru*
no journal, ,
The adsorption enthalpy of the Sg dioxydichloride on a quartz surface ( (SgO
(SgO Cl
Cl )) has been reported to be -98 kJ/mol. The result, however, is still ambiguous because the value was evaluated based on a few experimental points with large statistics errors. To obtain a reliable
)) has been reported to be -98 kJ/mol. The result, however, is still ambiguous because the value was evaluated based on a few experimental points with large statistics errors. To obtain a reliable  (SgO
(SgO Cl
Cl )) for a discussion of an influence of relativistic effects, a stable gas chemistry apparatus with good reproducibility is mandatory. In this study, we have conducted offline experiments of an isothermal gas chromatography) with short-lived Mo isotopes originated from a
)) for a discussion of an influence of relativistic effects, a stable gas chemistry apparatus with good reproducibility is mandatory. In this study, we have conducted offline experiments of an isothermal gas chromatography) with short-lived Mo isotopes originated from a 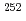 Cf fission source. We searched for experimental parameters for an on-line experiment. At the presentation, we will present the obtained optimum conditions for gas chromatographic separation of group-6 elements and determined
Cf fission source. We searched for experimental parameters for an on-line experiment. At the presentation, we will present the obtained optimum conditions for gas chromatographic separation of group-6 elements and determined  (MoO
(MoO Cl
Cl )).
)).
Natori, Hina; Sato, Tetsuya; Asai, Masato; Ito, Yuta; Uchibaba, Yuta; Gong, G.; Tsukada, Kazuaki; Miyachi, Yuta; Nagame, Yuichiro*
no journal, ,
To elucidate the chemical properties of element 106, seaborgium (Sg), we have constructed an off-line isothermal gas chromatographic system. As a model experiment for Sg using the system, gas-phase chemical experiments were performed for volatile oxychlorides of short-lived molybdenum (Mo) isotopes, a homolog of Sg, produced in spontaneous fission of 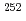 Cf. The optimum experimental conditions were obtained for isothermal gas chromatographic experiments using this apparatus. The observed isothermal gas chromatographic behaviors of short-lived Mo isotopes, we have successfully determined values of the adsorption enthalpies of Mo oxychlorides on the column surface. These values were in good agreement with those estimated from the sublimation enthalpies of the compounds.
Cf. The optimum experimental conditions were obtained for isothermal gas chromatographic experiments using this apparatus. The observed isothermal gas chromatographic behaviors of short-lived Mo isotopes, we have successfully determined values of the adsorption enthalpies of Mo oxychlorides on the column surface. These values were in good agreement with those estimated from the sublimation enthalpies of the compounds.
Natori, Hina; Sato, Tetsuya; Asai, Masato; Ito, Yuta; Uchibaba, Yuta; Gong, G.; Tsukada, Kazuaki; Miyachi, Yuta; Nagame, Yuichiro*; Otani, Ryo; et al.
no journal, ,
We have conducted gas-phase chemical experiments to investigate the volatilities of group-6 compounds as a model experiment for element 106, seaborgium (Sg). In this study, an isothermal gas chromatographic (IGC) technique was employed for off-line and on-line gas phase studies of volatile oxychlorides of group-6 elements, molybdenum (Mo) and tungsten (W). The volatile oxychloride formation of Mo and W was examined under various conditions using their short-lived isotopes. Moreover, several isothermal gas chromatograms were obtained for the compounds. The evaluated - values were in good agreement with the estimated value for MoO
values were in good agreement with the estimated value for MoO Cl
Cl and WO
and WO Cl
Cl , respectively. We concluded that only MO
, respectively. We concluded that only MO Cl
Cl (M=Mo, W) would be formed under the studied condition, and that adsorption enthalpies of MoO
(M=Mo, W) would be formed under the studied condition, and that adsorption enthalpies of MoO Cl
Cl and WO
and WO Cl
Cl were successfully obtained.
were successfully obtained.
 for liquid phase chemistry of element 106, Sg
for liquid phase chemistry of element 106, SgMiyachi, Yuta; Sato, Tetsuya; Tsukada, Kazuaki; Asai, Masato; Ito, Yuta; Natori, Hina; Nagame, Yuichiro*
no journal, ,
For the liquid phase chemistry of element 106, seaborgium, anion exchange experiments using tungsten ( W,
W, 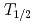 = 121 d) as its lighter homolog have been performed in HF/(0.1 M HNO
= 121 d) as its lighter homolog have been performed in HF/(0.1 M HNO ). It was found that the chemical equilibrium in the anion-exchange process would reach in a few minutes. The distribution coefficients of W on the anion-exchange resin were measured as a function of the HF concentration ([HF]). The results suggested the different chemical species of W in [HF]
). It was found that the chemical equilibrium in the anion-exchange process would reach in a few minutes. The distribution coefficients of W on the anion-exchange resin were measured as a function of the HF concentration ([HF]). The results suggested the different chemical species of W in [HF]  10
10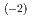 M and [HF]
M and [HF]  10
10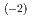 M.
M.
Natori, Hina; Sato, Tetsuya; Asai, Masato; Ito, Yuta; Tsukada, Kazuaki; Miyachi, Yuta; Nagame, Yuichiro*
no journal, ,
To elucidate the chemical properties of element 106, seaborgium (Sg), by gas-phase chemical separation technique, model experiments using its homolog molybdenum (Mo) were carried out. The adsorption enthalpies of the volatile compounds were obtained from the isothermal gas chromatographic behavior examined under various chlorination conditions for 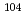 Mo (half-life
Mo (half-life 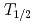 = 60 s),
= 60 s), 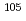 Mo (
Mo (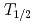 = 36.3 s) and
= 36.3 s) and 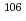 Mo (
Mo (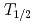 = 8.73 s). We have deduced the chemical species of the volatile oxychlorides observed in the separation by comparing the obtained values of the adsorption enthalpy with those estimated from the macro amounts.
= 8.73 s). We have deduced the chemical species of the volatile oxychlorides observed in the separation by comparing the obtained values of the adsorption enthalpy with those estimated from the macro amounts.